Gas In Solid Solution Example
Solution of Gas in Solid
Gases are always taken up by solids but the extent of uptake varies widely. It the uptake is by adsorption only the uptake is quite pocket-sized and this miracle in full general cannot exist taken every bit solution. In that location are iii other means in which the gases can exist taken up past solids:
(i) A gas may be distributed uniformly giving rising to a homogeneous solution in such a mode that at that place is no alter in the molecular structure or composition of the gas. Such cases institute true solutions and they resemble the solution of gas in liquid closely. Solutions of ammonia and sulphur dioxide on charcoal are examples of such cases. There is a decrease in the translational Kinetic energy of the molecules while they dissolve in the solid and such solutions are exothermic in nature. These systems obey Henry'due south law like the solutions of gas in liquid. If P be the force per unit area at which the gas is dissolved at a constant temperature then the concentration of the gas in the solution is directly proportional to the pressure. If the concentration of the gas in the solid is C, then P/C = constant and a plot of P vs C volition be a straight line, as shown in Figure one. If the gas undergoes every bit sociation in the solution and if n is the degree of association, then, P/n√C = abiding, whereas in case of dissociation the relation becomes, P/Cn = constant, where north now denotes the number of parts into which the molecule is dissociated.
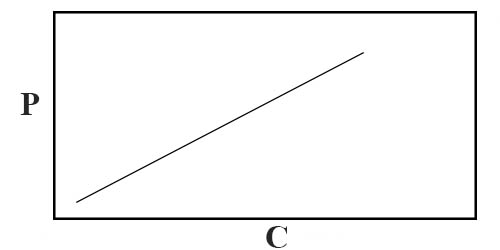
Figure: P vs C for solution of gas in solid
(ii) If the gas forms a solid solution a different beliefs is observed as shown in Effigy 2.The part ab represents the usual true solution and the relation P/C = K holds until the indicate b is reached. At b and up to c, a new solid solution is formed. Since the bend is an isothermal the system is invariant. Therefore, bc runs parallel to the pressure axis. On increasing the pressure still, further, this solid solution disappears at t and the usual relation P/C = Thou is followed along cd except that the directly line does not laissez passer through the origin.
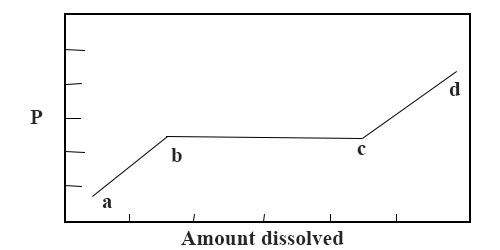
Fig: P vs amount of gas dissolved in solid
(iii) The third type of uptake of a gas by a solid is due to the chemical compound formation and no general conclusions are possible unless the dissociation pressure of the solid compound so formed is considered. Thus uptake of carbon dioxide by calcium oxide is an case of compound formation giving calcium carbonate.
Gas In Solid Solution Example,
Source: https://qsstudy.com/solution-gas-solid/
Posted by: funketingettere.blogspot.com


0 Response to "Gas In Solid Solution Example"
Post a Comment